Non Conformance Cause Category

This program lets you create and maintain the causes of non-conformances, so that you can prevent future issues, analyze trends and ultimately improve processes.
Exploring
This program is accessed from the Program List of the SYSPRO menu:
- Program List > Quality Management > Non Conformance Reporting > Setup
In non-conformance reporting, cause categories help classify the risks or reasons behind non-conformances, enabling organizations to identify patterns and implement effective corrective actions. By categorizing the causes of non-conformances, organizations can better analyze trends, improve processes, and prevent future issues. Some common cause categories are:
-
Human Error
Mistakes made by individuals during operations, such as miscommunication, inadequate training, or failure to follow procedures.
-
Process Failure
Breakdowns or inefficiencies in established processes, including inadequate controls, insufficient procedures, or lapses in quality checks.
-
Material Deficiency
Issues related to raw materials or components, such as substandard quality, improper specifications, or defects from suppliers.
-
Equipment Failure
Breakdowns or malfunctions of machinery or tools, which may arise from inadequate maintenance, wear and tear, or design flaws.
-
Environmental Factors
External conditions affecting production or product quality, including temperature, humidity, or contamination from the surroundings.
-
Design Issues
Flaws in the design or specifications of a product that lead to non-conformance, such as incorrect dimensions or inadequate functionality.
-
Regulatory Non-Compliance
Failures to meet legal or industry standards, resulting in products or processes that do not adhere to necessary regulations.
-
Documentation Errors
Problems stemming from inadequate or incorrect documentation, such as missing instructions, outdated procedures, or incorrect labels.
The non-conformance process is a systematic procedure used by organizations to identify, document, analyze, and resolve deviations from established standards, specifications, or regulations.
This process typically involves several steps, including the initial detection of a non-conformance, the creation of a non-conformance report, root cause analysis, implementation of corrective and preventive actions, and verification of effectiveness. The goal of the non-conformance process is to ensure that issues are addressed promptly, preventing recurrence and promoting continuous improvement in quality and compliance.
Starting
-
The Quality Management module must be installed.
You can restrict access to the eSignature transactions within a program at operator, group, role or company level (configured using the Electronic Signature Configuration Setup program).
Electronic Signatures provide security access, transaction logging and event triggering that gives you greater control over your system changes.
Controls access to the creation of a new cause category in the Non Conformance Cause Category program.
Controls access when maintaining a cause category in the Non Conformance Cause Category program.
Controls access when deleting a cause category in the Non Conformance Cause Category program.
You can restrict operator access to programs by assigning them to groups and applying access control against the group (configured using the Operator Groups program).
You can restrict operator access to programs by assigning them to roles and applying access control against the role (configured using the Role Management program).
The following configuration options in SYSPRO may affect processing within this program or feature, including whether certain fields and options are accessible.
To use this feature, the following setup option(s) must be enabled/defined:
Setup Options > Keys > Manufacturing
-
-
Suppress leading zeros
-
Presentation length
Solving
Why don't you drop us a line with some useful information we can add here?
Using
-
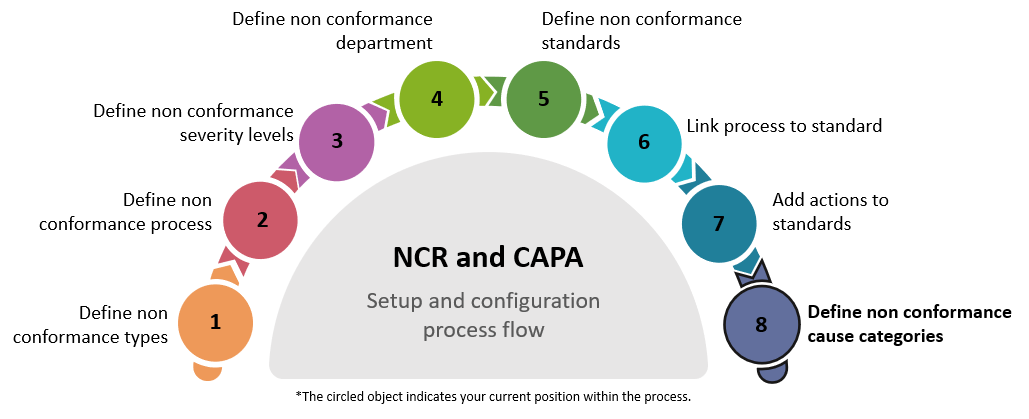
Define the non conformance types using the Non Conformance Type program.
-
Define the process that will be followed once a non-conformance is raised using the Non Conformance Process program.
-
Define the severity levels that will apply to the non conformance using the Non Conformance Severity Level program.
-
Use the Non Conformance Department program to define the departments that will own, address and resolve the non conformance issue, and to add both the default and any other necessary stakeholders for each department.
-
Define the non conformance standard using the Non Conformance Standard program by linking the type, severity level and departments to the standard.
-
Link the process to the standard using the Non Conformance Process Per Standard program
-
Link the corrective and preventative actions to the standard using the Non Conformance Standard Actions program.
-
Define the cause categories into which the non conformance incidents can be grouped using the Non Conformance Cause Category program.
Referencing
This lets you enter a new or select an existing cause category code used by non conformance reporting.
Non conformance cause categories are saved in the SqmCategory table.
| Field | Description |
|---|---|
|
Cause category |
This indicates the unique code for the NCR cause category. |
|
Description |
This lets you enter a description for the cause category. |
Copyright © 2026 SYSPRO PTY Ltd.